Acids and Bases
For All Chemistry Study Material PDF – Click Here
For All Subject Study Materials – Click Here
Acids and Bases are the main categories of chemical compounds. They have certain definite properties.
Acid:
- The word ‘acid’ is derived from a Latin word, which means “sour”.
- Organic acids: These acids contain carbon as a constituent and are present in organic matter i.e, animals and plants. For example Citric acid, acetic acid, tartaric acid etc. Organic acids are weak acids.
- Mineral Acids: These acids are prepared from minerals present in the Earth’s crust. For example sulphuric acid, hydrochloric acid , nitric acid,etc. These are also called laboratory acids. Mineral acids are strong acids.
- Sulphuric acid is known as the king of chemicals.
Base:
- Bases are substances that, in aqueous solution, release hydroxide (OH−) ions, are slippery to the touch, can taste bitter eg: Milk of Magnesia,baking soda, washing soda, calcium hydroxide, etc.
Weak and Strong Bases:
Strong bases: Some bases are readily soluble in water. These are strong bases. These are also called alkalies.eg: sodium hydroxide and potassium hydroxide.
- Strong bases are very corrosive and can burn the skin
Weak bases: Some bases are insoluble or partly soluble in water such bases are called weak bases.eg: ammonium hydroxide, calcium hydroxide etc.
Arrhenius concept of acids and bases:
- Acid is a substance which produces hydrogen ions in aqueous solution e.g. HCL (H+ Cl–), Sulphuric Acid,H2SO4(2H+ SO2-4).
- Base is a substance which produces Hydroxide ion (OH–) in aqueous solution e.g. sodium hydroxide and ammonium hydroxide etc.
Lewis concept of Acids and Bases:
- An acid is a substance which can accept an electron e.g. boron fluoride (BF3) and carbon dioxide.
- Base is a substance which can produce an electron e.g. fluoride (F–) and chloride (Cl–).
Bronsted Lowery concept of Acids and Bases
- An acid is a molecule or ion which is capable of donating a proton.
- A base is a molecule or ion which is capable of accepting a proton.
Some important acids:
| Acid | Present in |
|---|---|
| Acetic Acid | Vinegar |
| Ascorbic Acid | Amla |
| Citric Acid | Citrus Fruits |
| Formic Acid | Red ants |
| Lactic Acid | Curd |
| Tartaric Acid | Grapes, Ripe Mangoes |
| Oxalic Acid | Tomato |
| Malic Acid | Apple |
| Hydrochloric Acid | Stomach |
| Butyric Acid | Butter |
| Stearic Acid | Fat |
| Amino Acid | Protein |
| Tanic Acid | Tea |
Some important bases:
| Name of Base | Found in |
|---|---|
| (i) Calcium hydroxide | Lime water |
| (ii) Ammonium hydroxide | Window cleaner |
| (iii) Sodium hydroxide | Soap |
| (iv) Potassium hydroxide | Soap |
| (v) Magnesium hydroxide | Milk of Magnesia |
PH Scale:
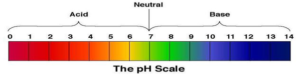
- PH value is a measure of the acidity or basicity of an aqueous solution.
- Solution with PH value less than 7 is considered as acidic.
- Solution with PH value greater than 7 is considered as basic.
PH values of some important solutions:
| Substance | P H Value |
|---|---|
| Hcl | 2.0 |
| Vinegar | 2.4 -3.4 |
| Wine | 2.8-3.8 |
| Beer | 4.0-5.0 |
| Lysosomes | 4.5 |
| Coffee | 4.5-5.5 |
| Human skin | 4.7 |
| Urine | 5.5-7.5 |
| Saliva | 6.5-7-5 |
| Tears | 7.4 |
| Blood | 7.3 to 7.5 |
| Pure water | 7 |
| Sea water | 8.5 |
| Ammonia | 12 |
Application of Acids:
| Acid | Uses |
|---|---|
| Hydrochloric acid | used for cleaning sinks and sanitry wares and in textile industry as a bleaching agent. |
| Nitric acid | used in manufacture of fertilizers, paints, explosives |
| Tartaric acid | used in making baking powder by mixing it with baking soda. |
| Citric acid | medicine |
| Acetic acid | used in preservation of food and for enhancing flavor of food. |
| Sulphuric acid | used in the batteries for cars, inverters |
Application of Bases:
| Base | Uses |
|---|---|
| Calcium hydroxide | used as an ingredient in whitewash, neutralizing acidic soil, in making bleaching powder and softening hard water. |
| Magnesium hydroxide | also known as milk of magnesia is used as an antacids. |
| Sodium hydroxide | also known as caustic soda is used manufacture of soaps, paper and textile. |
| Aluminium hydroxide | used as foaming agent in fire extinguishers. |
| Ammonium hydroxide | used in household cleaners and in fertilizers |
| Sodium carbonate+sulphuric acid | used in fire extinguisher. |

